Kieber-Emmons Group Research
The Kieber-Emmons group works at the nexus of inorganic, biological, and physical chemistry. Our research is focused on two main scientific interests: i) long-range proton transfers (LRPT) in respiratory oxidases, and ii) bioinspired catalysis with late transition metal complexes. Towards LRPT, our lab is developing a new, relatively non-perturbative methodology and associated instrumentation for the study of protein dynamics. The methodology will probe ns – ms changes in the electrostatic environment and correlate these changes to protein motion with capability to study systems under single turnover conditions. We use genetically incorporated unnatural amino acid vibrational probes and a unique time resolved hybrid vibrational spectrometer to provide detailed information on changes in the local electrostatic environment within a protein.
In efforts towards bioinspired catalysis, we were initially concerned with the mechanism of water oxidation by copper catalysts. We identified several lines of evidence that suggested di-copper rather than single site copper mechanisms are operative, which goes against the consensus paradigm. We have expanded on this concept by defining the bond dissociation free energies of several mono- and di-copper hydroxo complexes and the geometric and electronic structure origins of these fundamental parameters. This work has transitioned into proton induced O-O bond cleavage, O-O bond formation, and CH bond activation.
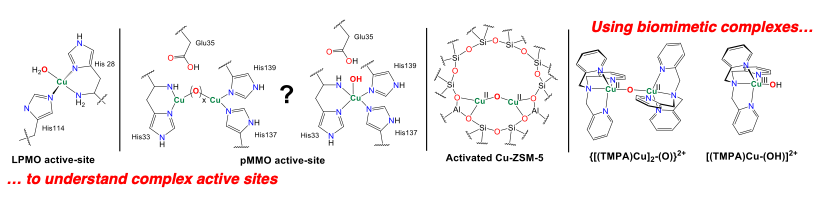
Our lab is highly interdisciplinary and a rich training environment for students. Students learn technical skills in molecular biology, synthesis, spectroscopy, and computations, but more importantly, they think critically about broad connections between fields. Our research is intrinsically collaborative, and we promote supportive and inclusive working environments.
Click here for our publications!